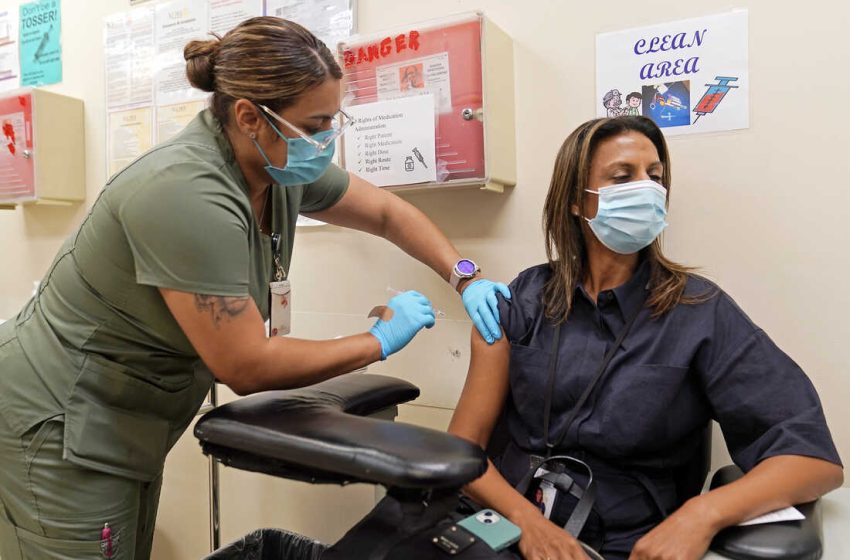
London, Europe Brief News – The US is poised to make COVID-19 vaccinations more like a yearly flu shot.
The decision is considered a major shift in strategy despite a long list of questions about how to best protect against a still rapidly mutating virus.
The Food and Drug Administration asked its scientific advisers to help lay the groundwork for switching to once-a-year boosters for most Americans — and how and when to periodically update the shots’ recipe.
“This is a consequential meeting to determine if we’ve reached the point in the pandemic that allows for simplifying the use of current COVID-19 vaccines,” said FDA’s Dr. David Kaslow.
The advisory panel mostly agreed with the FDA’s approach.
COVID-19 vaccines have saved millions of lives and booster doses continue to help the most vulnerable even as more contagious variants have popped up. But protection does wane and the shots don’t fend off milder infections for long.
And people are tired of getting vaccinated. While more than 80% of the U.S. population has had at least one COVID-19 shot, only 16% of those eligible for the latest boosters — so-called bivalent doses updated to better match more recent virus strains — have gotten one.
The FDA would have to decide how to phase in that change.


